
Can Axolotl Regeneration Genes Make Humans Immortal?
Think about this for a second. What if you lost an arm and just watched it grow back, perfectly, in a little over a month? For us, that is pure science fiction. We just grow up knowing that if a body part is gone, it is gone forever. Our biology is okay at patching up small cuts, but we usually walk away with a scar instead of real tissue.
Our bodies are basically hard-wired to close a wound as fast as possible. It is a survival trick, sure, but it stops us from rebuilding what we lost. The axolotl, though, plays by a whole different set of rules. These salamanders are legends in the world of regenerative medicine for a very good reason. They can regrow entire limbs, spinal cords, and even parts of their hearts with zero trouble.
To understand how wild this really is, an axolotl can grow a fully working leg back in about 40 days. This tiny water creature has a talent that seems to break every law of nature we have on the books. When it loses a limb, it doesn’t just heal over the stump. It builds a perfect copy. This is why researchers have spent decades obsessed with Axolotl Regeneration Genes.
The goal now is to figure out this process at a molecular level. Recent breakthroughs in genetic engineering have turned this from a curious hobby into a massive scientific question. Could we eventually use these same genetic shortcuts to help people heal faster or repair failing organs? It all comes down to how these animals handle their DNA.
When an injury happens, a specific group of Axolotl Regeneration Genes turns on. These genes tell normal cells to basically go back in time and act like stem cell therapy sources. This forms a mass called a blastema. You can think of it as a busy biological construction site where brand new tissues are built from zero.
In this article, we are diving into the real science. We will see if it is actually possible to put these powers into human DNA. We will also look at the lab results, the huge technical gaps, and the messy ethical fights that come with this research. By the end, you will see what is real and why the idea of human immortality is way more complicated than it sounds. Maybe the secret to healing is just hidden in the genes of a creature no bigger than your hand.
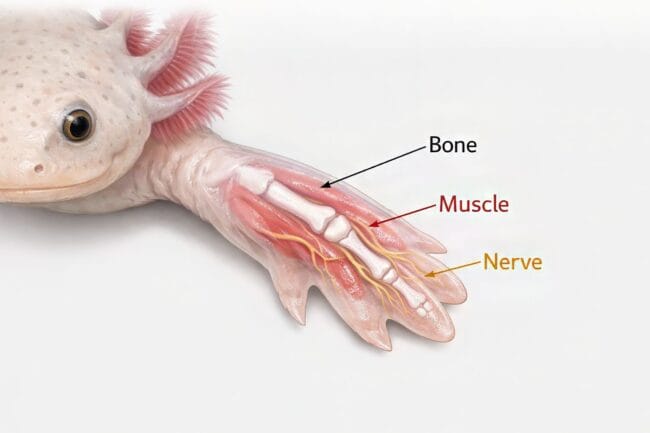
The Biology of Recovery: How Axolotls Regrow Limbs
The way an axolotl handles a serious injury is nothing short of a miracle. While a human body rushes to cover a wound with scar tissue to prevent infection, the axolotl takes a much more sophisticated path. Instead of just patching things up, it begins a systematic reconstruction of every missing part. This includes bone, muscle, nerves, and even blood vessels. Every piece is put back exactly where it belongs, restoring full structure and function to the limb.
The Secret Role of Axolotl Regeneration Genes
Everything starts at the genetic level. The moment an injury occurs, a specific group of Axolotl Regeneration Genes wakes up. These genes act like project managers on a construction site. They coordinate a very tight sequence of events that prevents scarring and instead promotes growth. Scientists believe that by studying these Axolotl Regeneration Genes, we might find a way to “remind” human cells how to grow again, rather than just forming a scar.
Step-by-Step Reconstruction: From Wound to Blastema
The process follows a very specific, choreographed routine:
- Wound Closure: Within hours, the axolotl covers the injured area with specialized skin cells. This isn’t just a scab. It is a signaling center that tells the body to start the repair work.
- Formation of the Blastema: This is the most critical stage. A mass of stem-like cells, called a blastema, forms under the new skin. These are essentially blank-slate cells ready to become whatever is missing.
- Differentiation and Growth: Under the guidance of Axolotl Regeneration Genes, these cells begin to differentiate. Some become bone, while others turn into muscle or nerves. They follow a biological blueprint until the limb is perfectly restored.
For researchers, this is not just a fascinating trick of nature. The axolotl is a living blueprint for complete tissue restoration. It shows us that perfect healing is biologically possible, provided you have the right genetic instructions to manage the job.
The Human Barrier: Why Can’t We Regrow Limbs?
If these Axolotl Regeneration Genes are so effective, it makes you wonder why humans didn’t keep this ability during evolution. We share a surprising amount of DNA with these salamanders, yet our bodies chose a different path. When we get hurt, our system is programmed for speed. We close wounds with scar tissue as fast as possible to stop infections. It is a survival trade-off. We traded the ability to regrow a leg for the ability to heal a cut quickly and keep moving.
Scarring vs. Regeneration
The biggest obstacle for us is fibrosis, or what we usually call scarring. In humans, specialized cells called fibroblasts rush to an injury and create a tough, collagen-rich patch. This patch is great for sealing the body, but it acts like a wall that blocks any chance of regeneration. The Axolotl Regeneration Genes that prevent this scarring in salamanders seem to be either missing or “switched off” in the human genome.
The Risk of Uncontrolled Growth
There is also the scary side of the conversation: cancer. Regeneration involves massive cell division, which is very similar to how tumors grow. Evolution might have suppressed our version of Axolotl Regeneration Genes to protect us from uncontrolled cell growth. In our quest for immortality, we have to figure out how to wake up these genes without accidentally triggering cancer. It is a incredibly delicate balance that scientists are still trying to understand in the lab.
The Molecular Engines: Key Genes Driving Axolotl Healing
The axolotl does not just magically grow back its body. It follows a strict set of genetic instructions. There is a small, elite group of genes that act like the primary drivers for this healing program. These are not just random pieces of DNA. They are the same pathways that built the animal when it was still inside its egg. In adults, these programs usually go to sleep, but in the axolotl, they stay ready to wake up at a moment’s notice.
The Masters of Plasticity: Msx1 and the Lin28 Axis
For regeneration to work, cells have to become flexible again. This is where genes like Msx1 come into play. This gene is famous for its ability to help cells lose their “adult” identity and revert to a more youthful state. Along with the Lin28 axis, these factors push cells to multiply rapidly. Without these specific Axolotl Regeneration Genes, the body would just produce a scar instead of a new limb. They are essentially the “fountain of youth” for individual cells.
Reawakening Growth: The FGF and Wnt Pathways
Think of FGF and Wnt as the power lines of the regeneration process. These pathways are responsible for sending signals that guide patterning and growth. In most vertebrates, once you reach adulthood, these developmental programs are locked away. However, the axolotl has a way to reawaken them. These signals tell the new cells exactly where to go and what to become, ensuring that a hand grows at the end of an arm and not somewhere else.
The Safety Switch: The p53 Pathway
Growing new tissue at such a high speed is dangerous. It is very similar to how cancer spreads. This is why the p53 pathway is so important. While we usually know p53 as a tumor suppressor, in the axolotl, it helps balance fast growth with genomic integrity. It acts like a safety inspector on a high speed construction site, making sure that as the Axolotl Regeneration Genes drive growth, the DNA remains stable and error free. This is likely why axolotls almost never get cancer, even though their cells are constantly dividing.
The Mystery of the Missing Scar: How Axolotls Avoid Fibrosis
In the human world, a deep cut almost always leaves a permanent mark. Our bodies are basically programmed to prioritize speed over everything else, so they rush to fill any gap with tough, fibrous tissue to keep infections out. But the axolotl plays by a completely different set of rules. This little salamander has a healing process that somehow skips the whole scarring phase. By digging into how they do this, scientists are hoping to learn how to flip a switch in humans. They want to move us from just “patching up” a wound to actually “rebuilding” what was lost.
A Different Kind of Immune Response
The real secret is hidden in the axolotl’s immune system. In mammals like us, our immune response is often way too aggressive. It rushes to build a thick wall of collagen at the injury site, which we call a scar. But the axolotl takes a much more relaxed approach. Instead of hitting the wound with intense inflammation, their system keeps things quiet. This calm environment is exactly what Axolotl Regeneration Genes need to take over. These genes make sure the body stays focused on growing back functional tissue instead of just dumping a pile of useless collagen into the hole.
The Role of Macrophages and Matrix Remodeling
The real stars of this show are the macrophages, which are a specific kind of white blood cell. In axolotls, these cells act more like expert site managers than just a cleanup crew. They are masters of what we call extracellular matrix remodeling. This means they are constantly managing the “scaffolding” of the tissue, making sure everything stays flexible and ready for new growth. This perfectly timed inflammation is the only reason they don’t get stuck with a scar. Without that tough scar tissue in the way, Axolotl Regeneration Genes can follow their original map and rebuild the limb perfectly.
Why This Matters for Us
Figuring out how axolotls manage to stop scars from forming is probably the most important piece of the puzzle. Right now, scar tissue is like a giant wall that stops human regeneration in its tracks. It is basically a locked door for our cells. If we can ever find a way to use Axolotl Regeneration Genes to create this same scar free environment in humans, it would change everything. We could finally help people recover from massive injuries or organ damage that everyone once thought was permanent.
Cellular Dedifferentiation Explained: Turning Back the Clock
The most mind-blowing part of axolotl biology is how their cells can actually “reverse” their own age. In humans, once a cell becomes a skin cell or a muscle cell, it is pretty much stuck in that role forever. But axolotls have figured out a way to break this rule through a process called dedifferentiation. It is essentially a biological time machine that allows mature cells to return to a much younger, more flexible state.
The Engine of the Blastema
So, how does the axolotl actually build a blastema? It doesn’t just wait for new cells to appear. Instead, it takes the existing, mature cells at the injury site and tells them to “forget” what they are. This process provides a steady supply of progenitor cells that can form many different types of tissue. It is all managed by Axolotl Regeneration Genes, which act like a reset button for cellular identity. Without this specific genetic trigger, the body wouldn’t have the “raw materials” it needs to start the rebuilding process.
Safe Growth vs. Chaos
One thing people often get wrong is thinking that this is just uncontrolled cell growth. In fact, dedifferentiation is a very carefully regulated process. It is not the same as the chaotic cell division you see in something like cancer. The axolotl manages to return its cells to a state of “plasticity” while still keeping their original lineage information intact. This means the cells remember their history even as they start new growth. Researchers are now obsessed with finding the triggers for this safe version of dedifferentiation. They believe that if we can understand these Axolotl Regeneration Genes better, we might find a way to safely replicate this process in human medicine.
The Cancer Paradox: Why Rapid Growth Doesn’t Lead to Tumors
Whenever cells start dividing as fast as they do during axolotl regeneration, it usually spells trouble. In humans, that kind of rapid growth is often the first step toward cancer. But here is the crazy part: axolotls are almost completely resistant to tumors. They can grow an entire limb in weeks, but they almost never get cancer. Scientists are looking at this paradox as a way to find a “safety switch” for human biology.
The Role of the p53 Pathway
It turns out that Axolotl Regeneration Genes have a built in security system. The most important player here is a gene pathway called p53. In our bodies, p53 is known as the “guardian of the genome” because it stops damaged cells from growing. In axolotls, this pathway is incredibly active during the rebuilding process. It acts like a high speed safety inspector. As the cells multiply to build a new arm, p53 makes sure that every single one of them is healthy and follows the rules.
Balancing Growth and Safety
This balance is exactly what makes Axolotl Regeneration Genes so special. They know how to push the “gas pedal” for growth while keeping one foot on the “brake” to prevent tumors. It is a level of genetic control that we just don’t have yet. If we can learn how the axolotl manages this, we might be able to treat cancer in a whole new way. Instead of just trying to kill cancer cells, we might learn how to reprogram them to behave like healthy, regenerating tissue. It is a total shift in how we look at medicine.
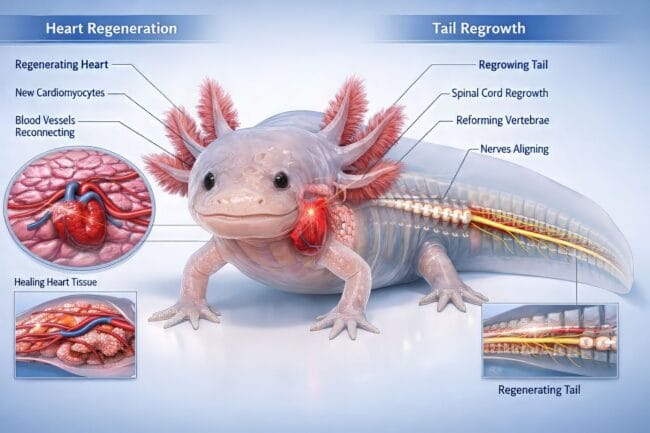
Beyond Limbs: Axolotl Tail and Heart Regrowth Explained
It is one thing to grow back a leg, but it is a whole other level of complexity to rebuild a spinal cord or a beating heart. Axolotls can do both with what looks like total ease. For humans, heart damage is usually a life sentence because our cardiac tissue is terrible at fixing itself. But for this salamander, an injury to the heart is just another problem to be solved. They can lose up to a third of their heart and simply grow it back without any loss of function.
The Mystery of Cardiac Rebuilding
Heart muscle regeneration is probably the most intriguing part of this research. While mammals form stiff scars after a heart attack, axolotls use specific Axolotl Regeneration Genes to trigger the growth of new, healthy muscle cells called cardiomyocytes. It is not just about growing the muscle back, though. They also have to reconnect blood vessels to make sure the new tissue actually works. This process of vascular reconnection is flawless in axolotls, ensuring the heart keeps beating perfectly throughout the entire repair.
The Tail and the Spinal Cord
The tail is another masterpiece of nature. When an axolotl regrows its tail, it is not just skin and muscle. It is rebuilding a complex structure that includes the spinal cord and vertebrae. This is a huge deal for medical science. By studying the Axolotl Regeneration Genes that manage tail regrowth, researchers are looking for clues on how to fix spinal injuries in humans. The way they manage to align every nerve and bone perfectly is a living blueprint for what we hope to achieve in future surgeries.
Why This Knowledge Matters for Us
Studying these signals that promote cell growth in the heart and spine is the key to new heart repair strategies. If we can understand how to “instruct” human heart cells to start multiplying again after an injury, we could save millions of lives. The axolotl shows us that the body has the potential to heal even its most vital organs. We just need to learn the right genetic language to unlock that ability in our own DNA.
Comparing Human and Axolotl Genes: Why the Difference?
You might be surprised to learn that humans and axolotls actually share a huge amount of DNA. We have many of the same “homologous” genes, which are basically genetic cousins. However, having the same parts doesn’t mean you get the same results. It is like having the same ingredients as a world-class chef but not knowing the secret recipe. The difference in how our bodies heal comes down to three things: regulation, timing, and the cellular environment.
Silent Genes and Different Wiring
The most fascinating part is that some Axolotl Regeneration Genes are actually present in the human body right now. The problem is that in us, they are either silent or “differently wired.” In a salamander, an injury wakes these genes up immediately. In a human, those same genes might stay asleep or even do something completely different, like creating a scar. Scientists are now looking for the molecular levers that would need to be adjusted to nudge human tissues toward a more regenerative path.
The Role of Epigenetics and Signaling
It is not just about the genes themselves, but also about the “switches” that turn them on or off. By comparing the epigenetic states of humans and axolotls, researchers are finding clues about why our cells refuse to go back to a youthful state. The signaling context in an axolotl wound is one of growth and repair, while in a human, it is one of defense and scarring. If we can learn how to change that signal, we might be able to coax our own bodies into following the axolotl’s blueprint. It is a massive challenge, but the more we compare our genetic codes, the closer we get to the answer.
Can Humans Regenerate Limbs? The Reality Check
If we are being honest, the answer is no, at least for now. You won’t see a human regrowing a full arm in a hospital anytime soon. That remains strictly in the world of science fiction. Our bodies just aren’t built for that level of repair yet. However, this does not mean we are completely without hope. Humans actually have several hidden regenerative abilities that we often don’t even notice. We are starting from a much better place than most people realize.
Small Successes: From Finger Tips to Organs
The evidence is already there if you look closely. For instance, young children have been known to regrow the tips of their fingers after an accident, provided the injury is treated correctly. It is a rare phenomenon, but it proves the potential is there. We also have a liver that can rebuild itself from just a small fragment, and our skin is constantly in a state of self-repair. These are small wins, but they show that the biological machinery is already in our DNA. Many of the same Axolotl Regeneration Genes have cousins in our own genome that are responsible for these minor miracles.
Closing the Gap Between Species
There is obviously a huge difference between fixing a fingertip and regrowing an entire limb. It is a massive gap that scientists are trying to bridge. By mixing new discoveries in biology with high tech biomaterials and better surgical methods, we are slowly pushing the limits of what the human body can do. The goal is to study Axolotl Regeneration Genes to find ways to guide our own cells. We want to create environments where human tissue feels “safe” enough to start growing again. It is a slow process of making small, steady gains, but every step forward brings us closer to a future where major injuries are no longer permanent.
CRISPR and the Future of Human Repair
If we ever want to actually use the axolotl’s secrets, we have to find a way to rewrite our own biological code. This is exactly why CRISPR is such a big deal. Think of it as a set of highly precise molecular scissors. It gives scientists the power to cut and paste DNA with a level of accuracy that was impossible just a decade ago. We are now at a point where we can start testing if these Axolotl Regeneration Genes can be “installed” into human cells to see how they react.
Testing the Master Plan
Researchers are not just using CRISPR to change DNA for the sake of it. They are using it to solve a massive biological puzzle. By switching specific genes on and off, they can finally see which ones are the real “engineers” behind a regrowing limb. Some teams are even trying to build human cell models that mimic the axolotl’s unique programs. This is the only way to truly understand Axolotl Regeneration Genes. If we can figure out which genetic switches to flip, we might be able to jumpstart the same kind of healing in our own bodies.
Why We Must Move Slowly
As amazing as CRISPR sounds, we have to be extremely careful. Messing with the instructions for cell growth is like trying to fix a plane while it is still flying. One wrong move and you could trigger something terrible, like cancer or other massive system failures. This is the dark side of gene editing that keeps scientists awake at night. The challenge is to use Axolotl Regeneration Genes to trigger repair without accidentally starting a chaotic, uncontrolled chain reaction. It is a very thin line between a medical miracle and a total disaster.

From Lab to Clinic: Human Trials Inspired by Axolotl Genes
Moving a discovery from a salamander’s tank to a human hospital is a long and difficult journey. It doesn’t happen overnight. Scientists usually have to go through several intermediate steps to make sure everything is safe. This usually involves testing axolotl-associated gene variants in mice or lab-grown “organoids,” which are essentially tiny versions of human organs. These experiments are crucial for finding the right therapeutic targets before we even think about treating a person.
The Current State of Human Testing
Right now, we are seeing a handful of early-stage trials that use stem cells or gene therapies to fix damaged tissue. However, it is important to be clear that we aren’t just injecting people with axolotl DNA yet. That has not become a standard treatment by any means. Instead, doctors are looking at how Axolotl Regeneration Genes can teach us to improve our own existing therapies. We are learning how to make human stem cells more effective by mimicking the signals that work so well for the axolotl.
The Big Hurdles: Safety and Delivery
Before these treatments can become common, we have to clear two massive hurdles. First, there is the issue of safety. Any time you talk about changing how cells grow, you have to do exhaustive testing to make sure you aren’t creating new problems. Second, we need scalable delivery methods. It is one thing to edit a few cells in a lab, but it is much harder to deliver that treatment to millions of patients. Understanding Axolotl Regeneration Genes gives us the map, but we still need to build the road to get there safely.
Building Life: Tissue Engineering and Bioengineering Organs
Having a DNA manual is all well and good, but cells still need an actual place to do their jobs. You can’t just throw a bunch of cells into a wound and expect a miracle. They need a solid foundation. This is exactly why tissue engineering is so vital. Right now, scientists are experimenting with things like 3D printing and bio-scaffolds to build a frame for new organs. It is a lot like building a house. You wouldn’t try to put up the wallpaper before you have the wooden beams in place, right?
Providing a Template for Real Growth
The real breakthrough happens when we mix these high tech frames with living cells. 3D printed templates act like a physical map, showing cells exactly where they need to go. In normal human healing, this map is usually missing, which is why we end up with scars. We are constantly digging into Axolotl Regeneration Genes to understand the kind of environment these cells actually prefer. If we can build a scaffold that mimics an axolotl’s body, we might finally talk human cells into rebuilding complex parts instead of just quitting and forming a scar.
The Nightmare of Wiring and Plumbing
But here is the catch that really frustrates researchers. Making a shape is the easy part. The real nightmare is the plumbing. A working organ is useless if it doesn’t have a blood supply or a way to talk to the brain. Right now, trying to engineer vascular and neural networks is a massive wall we keep hitting. A lump of tissue isn’t enough. It has to breathe and it has to react. This is why we are obsessed with Axolotl Regeneration Genes. We need to steal their trick for reconnecting nerves and blood vessels with such perfect accuracy. Without that secret, these bioengineered organs will never be more than just static models.
The Real Danger: Could We Accidentally Cause Cancer?
Let’s not sugarcoat it. If you start forcing human cells to grow at high speeds, you are basically playing a dangerous game with cancer. It is the biggest worry in this whole field of study. The scary part is that under a microscope, a regrowing limb and a spreading tumor actually look almost identical. Both involve cells that are dividing like crazy and simply refuse to die. If we want to use Axolotl Regeneration Genes to heal people, we have to be absolutely certain we aren’t just lighting a fire that we can’t put out later.
The Scary Similarity Between Healing and Tumors
This entire process is incredibly risky because of how rebuilding actually works. To grow back a missing part, cells have to become flexible and start multiplying as fast as possible. That is exactly the same strategy a tumor uses to take over. For any of this to ever work in a real hospital, we need more than just a way to start the growth. We need a massive emergency brake. We need a way to tell those cells exactly where to stop and when to shut the whole thing down. If we can’t control those limits, a healing arm could turn into a life threatening growth before we even know what hit us.
Stealing the Axolotl’s Safety Switch
The real secret we need to steal from these animals is how they keep their DNA from falling apart during the chaos of rebuilding. Axolotls are total experts at this. They can flip their Axolotl Regeneration Genes on, finish the job, and then lock the door behind them before things get out of control. Humans would need a similar “off switch” that is basically impossible to break. Any strategy for regrowing tissue that doesn’t have a bulletproof security system is just too risky to even try. We have to find a way to push the limits of medicine without losing our grip on the cells we are trying to save.

The Ethical Mess: Where Do We Draw the Line?
Rewriting human DNA to make us heal like salamanders sounds great, but it opens up a huge can of worms. We have to ask ourselves some really tough questions. Are we just fixing a problem, or are we trying to “upgrade” the human race? There is a very blurry line between medical therapy and genetic enhancement. If we start using Axolotl Regeneration Genes to change how our bodies work, we need to be very clear about who gets to decide what is “normal” and what is “extra.”
Who Gets to Be Superhuman?
One of the biggest worries is that these technologies might only be available to the rich. This could create a world where only a small group of people can regrow limbs or heal from major accidents, while everyone else is left behind. It is not just about the science. It is about fairness. We also have to think about the long term effects. If we change someone’s genes, those changes might be passed down to their children. We are basically making decisions for future generations without their permission.
Rules for a New Frontier
Our current laws and rules just aren’t ready for this kind of technology. As the science moves faster, our ethical frameworks are struggling to keep up. We can’t just let things happen and hope for the best. We need strong, clear guidelines that protect human rights and social values. Using Axolotl Regeneration Genes is a massive responsibility. We have to make sure that clinical trials are done with total honesty and that we never lose sight of the people we are actually trying to help. It is about more than just what we can do. It is about what we should do.
Let’s be real. Regrowing a limb or an organ isn’t just a medical win. It is a social bomb. If we actually manage to extend the human lifespan in a big way, our whole world is going to break. Every single system we have is built for people who get old and eventually die. If we suddenly have 120 year olds running around with the bodies of 30 year olds, everything changes. Our ideas about when to work and when to quit will be totally trashed. Is our society even close to being ready for that kind of mess?
The Nightmare of Never-Ending Jobs
Think about the job market for a second. It would be a total nightmare. If people stay young and healthy for a hundred years, they are never going to retire. This means the younger generation is going to be stuck at the bottom with nowhere to go. It is a recipe for a massive fight between the old and the young. And what about hospitals? Our healthcare is designed to fix sick people. But if we use Axolotl Regeneration Genes to stop aging and keep everyone healthy, the entire medical business model just vanishes. We are looking at a giant puzzle and we don’t have a single piece in place yet.
This Isn’t Just Some Movie Plot
People think this is just science fiction, but it is actually staring us in the face. We need to stop just looking at the cool biology and start talking to the people who run our cities and countries. If we keep ignoring the social fallout, we are headed for a lot of trouble. Using Axolotl Regeneration Genes to keep us alive longer is a huge power to have. But it could also make the world incredibly unfair if only the rich get to live forever. We have to make sure that living longer doesn’t just turn into a century long fight for survival.
Longevity vs Quality of Life: What Is the Point of Living Longer?
Let’s get one thing straight. Living for 150 years isn’t actually a good thing if you spend the last 70 of them in pain or unable to move. Extending life without making sure those extra years are healthy is basically pointless. The real goal of regenerative medicine shouldn’t just be about adding more birthdays to the calendar. It has to be about restoring the body so that people can actually enjoy their lives. We are looking for function, not just more time.
Focusing on the Years That Actually Count
When we talk about using Axolotl Regeneration Genes, our primary aim should be reducing suffering. It is about giving someone back their independence. Being able to walk, play with your grandkids, or just take care of yourself is much more important than any raw lifespan number. These are the metrics that actually matter to real people. If we can use the axolotl’s secrets to fix a damaged heart or a broken spine, we are improving the quality of life, which is the only thing that makes longevity worth having.
Setting Our Priorities Straight
Ethical and medical priorities need to reflect this reality. We can’t get so distracted by the idea of “living forever” that we forget about the people who are suffering right now. The science of Axolotl Regeneration Genes should be a tool for healing, not just a way to cheat death. We need to make sure our clinical goals are focused on making those extra years meaningful. A longer life is only a victory if it is a life that is actually worth living.
Next Steps in Regenerative Medicine: Where Do We Go From Here?
The path forward isn’t just about one big discovery. It is about a lot of small, difficult steps that have to happen at the same time. Our immediate goal is to map out exactly how different species, like the axolotl, manage to rebuild themselves. We need to understand those genetic networks inside and out. But even more importantly, we have to find better ways to actually deliver these treatments to human cells without causing chaos. It is a massive technical challenge that requires much more than just a good idea in a lab.
Better Tools for a Complex Job
To make this work, we need a whole new set of tools. We are looking at better biomaterials to support cell growth and smarter imaging to see what is happening deep inside the tissue. We also need computers that can help us model how cells behave before we even try a treatment on a person. It is about building a safer, more predictable way to control how our bodies heal. By studying Axolotl Regeneration Genes, we are learning the rules of the game. Now we just need the right equipment to play it properly.
The Power of Working Together
No single scientist is going to solve this on their own. The real progress is going to come from interdisciplinary teams. We need biologists who understand the cells, engineers who can build the scaffolds, and ethicists who can keep us grounded. Most importantly, we need clinical experts who know how to turn these theories into actual treatments for patients. Using Axolotl Regeneration Genes as a guide is a group effort. When we combine all these different types of expertise, we can accelerate progress in a way that is both fast and responsible.
Digital Healing: How AI and Genetics Predict the Future
We have so much data on genetics now that a human brain simply can’t process it all. This is where machine learning and bioinformatics come into play. These are powerful tools that help us predict which gene combinations are actually going to work. Instead of guessing, we use these systems to find the safest way to trigger regeneration. It is like having a supercharged search engine that can sift through millions of genetic possibilities to find the one that won’t cause dangerous side effects.
Finding the Best Path Without the Guesswork
Computational models are great because they can pull together massive amounts of information from different areas of biology. They help us identify which interventions are worth testing and which ones are likely to fail. AI doesn’t replace the actual lab work, of course. You still need real experiments to see what happens. However, it does help us prioritize the best leads. This means we can design much more efficient studies and avoid wasting time on ideas that were never going to work in the first place.
Using AI to Master the Axolotl Code
The real goal here is to use AI to finally crack the code of Axolotl Regeneration Genes. By feeding these machines data from axolotl DNA, we can start to see patterns that were invisible to us before. We can forecast how a human cell might react if we try to mimic those same patterns. It is about using technology to take the risk out of the science. With AI as our partner, we are moving from a “trial and error” approach to a much more precise way of healing the human body.
From Bench to Bedside: Translating Lab Findings to Clinics
Moving a discovery from a lab bench to a patient’s bedside is a massive undertaking. It is not just about the science. It is about making sure that the science is reproducible and that we can actually manufacture these treatments on a large scale. Many promising breakthroughs end up failing because we simply can’t find a way to deliver them to the right part of the human body. We also have to account for the fact that human biology is much more unpredictable than a lab model.
The Hurdle of Regulation and Human Biology
One of the biggest challenges is getting regulatory approval. This requires exhaustive testing and a clinical trial design that actually measures outcomes that matter to patients. It is a long and expensive process. To improve the odds of success, scientists need to engage with regulators early on and have very clear criteria for what counts as a win. Without this careful planning, even the most exciting research into Axolotl Regeneration Genes will never make it past the lab doors.
Building a Bridge to Real Therapies
To turn a lab finding into a real therapy, we have to solve the “delivery problem.” This means finding a way to get the genetic or cellular instructions exactly where they need to go without causing side effects. By focusing on translational planning from the very start, we can avoid some of the most common pitfalls. We are learning from the axolotl how to guide cells, but we still need the right clinical framework to apply those lessons safely to humans. The goal is to make sure these discoveries don’t just stay in a journal, but actually reach the people who need them.
Optimism with Realism: What the Axolotl Actually Teaches Us
The axolotl is honestly amazing. It gives us a rare look at what biology is capable of when it really gets to work. But let’s stay grounded here. These creatures aren’t offering us some magic shortcut to living forever. Real progress in this field is slow and it is difficult. It is about the gritty work of figuring out molecular patterns and taking very small, careful steps toward treatments that actually work in the real world. We need strict safety rules and serious ethical debates to make sure we don’t lose our way.
The Real Win Isn’t Immortality
We have to be honest about the goal. The most important endgame isn’t to stop death. It is about finding ways to fix a broken body and stop someone from suffering. If we can use Axolotl Regeneration Genes to help a person walk again or to repair a failing heart, that is a massive victory. That kind of progress is way more meaningful than just trying to add more years to a life that is full of pain. We should be chasing a better quality of life, not just a longer one.
Rebuilding Without Cheating Nature
If we move forward with a bit of humility, the axolotl will keep showing us the way. This isn’t about trying to cheat the system or break the limits of biology. It is about understanding those limits and learning how to work inside them to rebuild what was lost. These animals have been doing this for millions of years. We are just the students trying to learn their language. By being smart about the science and the ethics, we can turn these lessons into a future where an injury doesn’t have to be a life sentence.
The Future of Regeneration: How to Get Involved
Everything we are seeing in regenerative biology right now is changing the game. It is flipping the script on how we think about healing, getting older, and the whole future of medicine. If you are an investor, a researcher, or just someone building a business, this isn’t just another science project. It is a rare chance where massive scientific goals actually meet a huge demand in the real world.
Are you looking for a way into biotech or health innovation? Then you need to be paying close attention to this field right now. Don’t just sit on the sidelines. Follow the work being done in regenerative medicine and help push for responsible development. Get connected with the companies and the labs that are taking these discoveries and turning them into real treatments.
As always, ensure you conduct your own due diligence before making any professional or investment decisions. The future of healing is being built today, and you should be part of it.
Frequently Asked Questions: Can Axolotl Regeneration Genes Make Humans Immortal?
Q: Can humans actually regenerate like axolotls?
A: Not in the way we usually see in movies. Humans have very limited regenerative powers, like how children can sometimes regrow a fingertip or how our livers can repair themselves. However, regrowing a whole arm or a complex organ like an axolotl does is currently impossible for us. The whole point of current research is to use stem cells and Axolotl Regeneration Genes to see if we can eventually unlock that same potential in humans.
Q: Which specific genes are actually doing the heavy lifting?
A: Scientists have pointed to genes like Msx1 and Lin28-related factors, along with pathways called FGF and Wnt. These are the “engineers” that tell cells to stop being specialized and start multiplying to build new tissue. They are the reason an axolotl can grow back a heart or a limb without any scarring. Research focuses on activating these dormant pathways in human cells safely.
Q: Could these genes really make humans immortal?
A: That is a big “maybe,” but probably not in the way most people think. While these genes are great for repairing damage and maybe extending how long we stay healthy, immortality is a lot more complicated than just regrowing a part. Current science is focused on fixing injuries and improving our “healthspan,” not on bypassing aging entirely.
Q: What are the biggest risks of using these genes in humans?
A: The biggest fear is cancer. When you tell cells to grow fast and ignore the usual rules, you are walking a very thin line. There is also the risk of the immune system attacking the new tissue or the body just doing something totally unexpected. This is why we need massive amounts of safety testing and controlled therapies before this ever touches a human patient.
Q: How is this research being applied in medicine today?
A: Today, it is mostly about incremental steps. We are seeing stem cell therapies, tissue engineering, and gene therapy trials being used to heal wounds or try to fix damaged organs. We aren’t regrowing limbs in clinics yet, but the lessons we are learning from Axolotl Regeneration Genes are already helping us design better treatments for healing.
Q: Are there ethical concerns in using axolotl genes in humans?
A: Absolutely. It is a huge mess of questions. We have to decide where to stop. Is it okay to just fix a wound, or is it also okay to “upgrade” a human body? There is also the worry about equitable access. If only the rich can afford these treatments, it would create a massive social divide. We need real laws and ethical rules to keep this technology from getting out of hand.
Q: How far are we from practical human regeneration?
A: Lab experiments using model organisms and organoids are moving fast. However, translating these findings to full human limb or organ regeneration will likely require decades of research. It is one thing to make it work in a petri dish and a totally different thing to make it work safely in a living, breathing person. It will take many more clinical trials to get there.
Q: Can regeneration reduce the effects of aging?
A: It definitely could. Regeneration therapies may improve tissue repair and restore organ function, which would enhance our healthspan. While they could delay some aging effects, they do not currently stop the whole process of aging. The goal is to make those extra years more meaningful and active by keeping the body functional for longer.
Editorial Disclaimer
Let’s be very clear about this right from the start. This article is only here to look at the science behind axolotls and what that might mean for the human body. It is not a medical guide in any way. You should not use any of this information to try and diagnose yourself or to find a treatment for any condition. The topics we are talking about, like Axolotl Regeneration Genes and living longer, are still experimental and extremely complex. This science is simply not ready for people to use on their own.
You need to go and talk to a real doctor or a qualified scientist before you even think about making changes to your health or your genetics. Trying out experimental therapies is dangerous. The people who wrote this and the ones who published it are not responsible for what you decide to do with this information. We are just sharing what is happening in the labs. Your medical choices are your own and you should always work closely with professional healthcare providers.
References
- Advancements in Axolotl Models: A detailed overview of the cellular and molecular mechanisms linking regenerative capabilities with the aging process via National Center for Biotechnology Information (NCBI).
- The Genetic Odyssey of Axolotl (2024): A comprehensive genetic review highlighting recent innovations and discoveries in the study of axolotl regeneration via The International Journal of Developmental Biology (IJDB).
- Multi-species Transcriptional Atlas: A cross-species transcriptional comparison that resolves long-standing paradoxes in axolotl limb development and regeneration via Nature Communications.
- Axolotl Limb Blastema Mechanisms: Foundational research exploring the specific cellular and molecular pathways that govern blastema formation via PubMed Central (PMC).
- Regeneration Lessons from the Axolotl: A seminal academic review of regenerative programs and their potential implications for human medicine via ScienceDirect.
- Integrative Analysis of Gene Expression: An in-depth look at differential gene expression during the regeneration process using microarray and RNA-Seq data via Nature Scientific Reports.
- Positional Identity and Human Repair: Strategic research into positional identity and retinoic acid, providing a foundation for future work in human tissue repair via National Science Foundation (NSF).
